Want to see the full answer. THIS SET IS OFTEN IN FOLDERS WITH.

Solved Which Of The Following Subshells Does Not Exist O 4d Chegg Com
A 2d B 2s C 2p D all of the above E none of the above.
. Only 2s2p orbitals are present in 2nd shell. Only 3s 3p3d orbitals are present in 3rd shell. Which of the following subshells does not exist.
If the subshell cannot exist what is wrong. How many orbitals have the set of quantum numbers n4 and l2. Problem 39 Medium Difficulty.
91 Of the following only __________ is not a metalloid. All of the above e. Correct options are B and C Only 1s orbital is present in 1st shell.
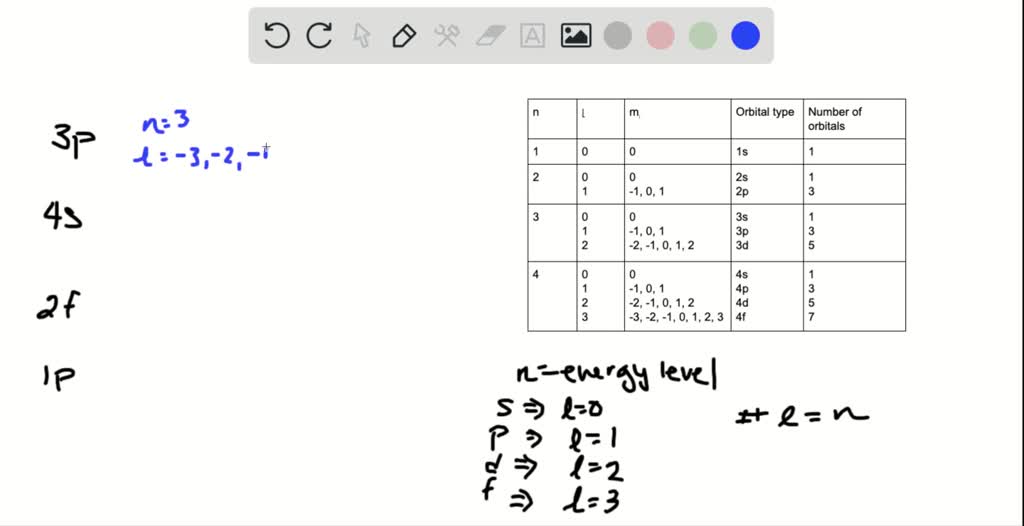
10Which one of the following is not a valid value for the magnetic quantum number of an electron in a 5d subshell. In which energy levels n1 to 4 can you find the following energy subshells. The value of subshells depends on the principal shell ie for given value of n we have corresponding l values but their values are n-1 as maximum.
Check out a sample QA here. Which one of the following is not a valid value for the magnetic quantum number of an electron in a 5d subshell. Who are the experts.
F d p s E lOMoARcPSD11635688 E. Which of the following subshells cannot exist in on atom. Some elements in periodic table are classified as metalloids as they have intermediate properties of both metals and nonmetalsThus metalloids are also called as semimetals.
Which of the subshells does not exist due to the constraints upon the angular momentum quantum number. In the 3rd energy level electrons occupy only the s p and d sublevels so there is no f sublevel. We review their content and use your feedback to keep the quality high.
Which orbital or orbitals are. A2 B1 C0 D3 E-1 10 11Which of the subshells below do not exist due to the constraints upon the azimuthal quantum number. A2p b2d c2s dall the above enone of the above.
Students whove seen this question also like. The Pauli exclusion principle states that. Which statement is correct based on periodic trends.
Only 4s 4p4d and 4f orbitals are present in 4th shell. 15 Which of the subshells below do not exist due to the constraints upon the azimuthal quantum number. Chemistry questions and answers.
Terms in this set 45 Which of the following subshells do not exist. A 6s B 3f C 3p D 2s E 4d. Chemistry QA Library Which of the following subshells do not exist.
2d 4f 3f all do not exist except 4f 1p 2d was not the answer 2. Which of the following subshells does not exist. Recall that the first two quantum numbers are.
Which of the following are possible energy levels that can be occupied by electrons. 1s 6For each of the following pairs circle the sublevel that is. State which of the following orbitals cannot exist according to the quantum theory.
For the given subshells 2d and 3f doesnt exist because for prinicipal value n 2 we have only 2 subs. Group of answer choices2s2pall of the above3fnone of the above Submitted. How many p orbitals are in the n4 shell.
Because those angular momenta are too high for the given quantum levels. Which of the following orbital occupancy designations is incorrect. Which of the following subshells does not exist.
A Si B As C B D Al E Ge. The number of orbitals in a p subshell is. In the third energy level the electrons are located only in the s and p subshell so a 2d subshell does not.
Aluminium is a metal. Want to see the full answer. Check out a sample QA here.
Which of the subshells below do not exist due to the constraints upon the angular momentum quantum number. Which of the subshells below do not exist due to the constraints upon the angular momentum quantum number. L 012n 1 spdfghik.
Which one of the following is not a valid value for the magnetic quantum number of an electron in a 5d subshell. 2 s 2 d 3 p 3 f 4 f and 5 s Briefly explain your answers. 3p 4d 3d 3f 6s Expert Solution.
See the answer See the answer done loading. Quantum Numbers Concept Videos All Chemistry Practice Problems The Electron Configuration. Which of the subshells below do not exist due to the constraints upon the angular momentum quantum number.
Which of the following subshells does not exist in atoms. Which of the subshells below do not exist due to the constraints upon the angular momentum quantum number. O 4d ОЗр O2s 06g O 34.
None of the above. 4f 4s all of them do exist 2p sd sd and 2p were not the. Which of the following subshells does not exista.
Up to 25 cash back Which of the subshells below do not exist due to the constraints upon the angular momentum quantum number. Neon has a higher ionization energy than fluorine b. A2s B2p C2d Dall of the above Enone of the above 11.
A 4 B 4 C 4 D 4 E none of the above Answer. All of the above subshells exist. Quantum Numbers Practice Problems.
It forms a tightly arranged crystal structure. Which of the following subshells does not exist. A 2 B 3 C 0 D 1 E -1.
4d Learn this topic by watching The Electron Configuration. The degree of supply chain responsiveness does not need to be consistent. Experts are tested by Chegg as specialists in their subject area.
Two types of pure substances are. The answer is b eq2d eq. Which of the following subshells does not exist.
No two electrons in the same atom can have the same set of four quantum numbers. A2d b4d c4g d6f By.

Which Of The Following Sub Shell Does Not Exist For An Atom According To Quantum Theory Youtube

Teaching Chemistry Atom Physics Formulas

Solved State Which Of The Following Orbitals Cannot Exist According To The Quantum Theory 3 P 4 S 2 F And 1p Briefly Explain Your Answers
0 Comments