Question 6 1 1 pts Which of the following best describes ionic bonding. Metal transfers or donates its electron to a non-metal forming an ionic compound B.

Ionic Bond Definition Properties Examples Facts Britannica
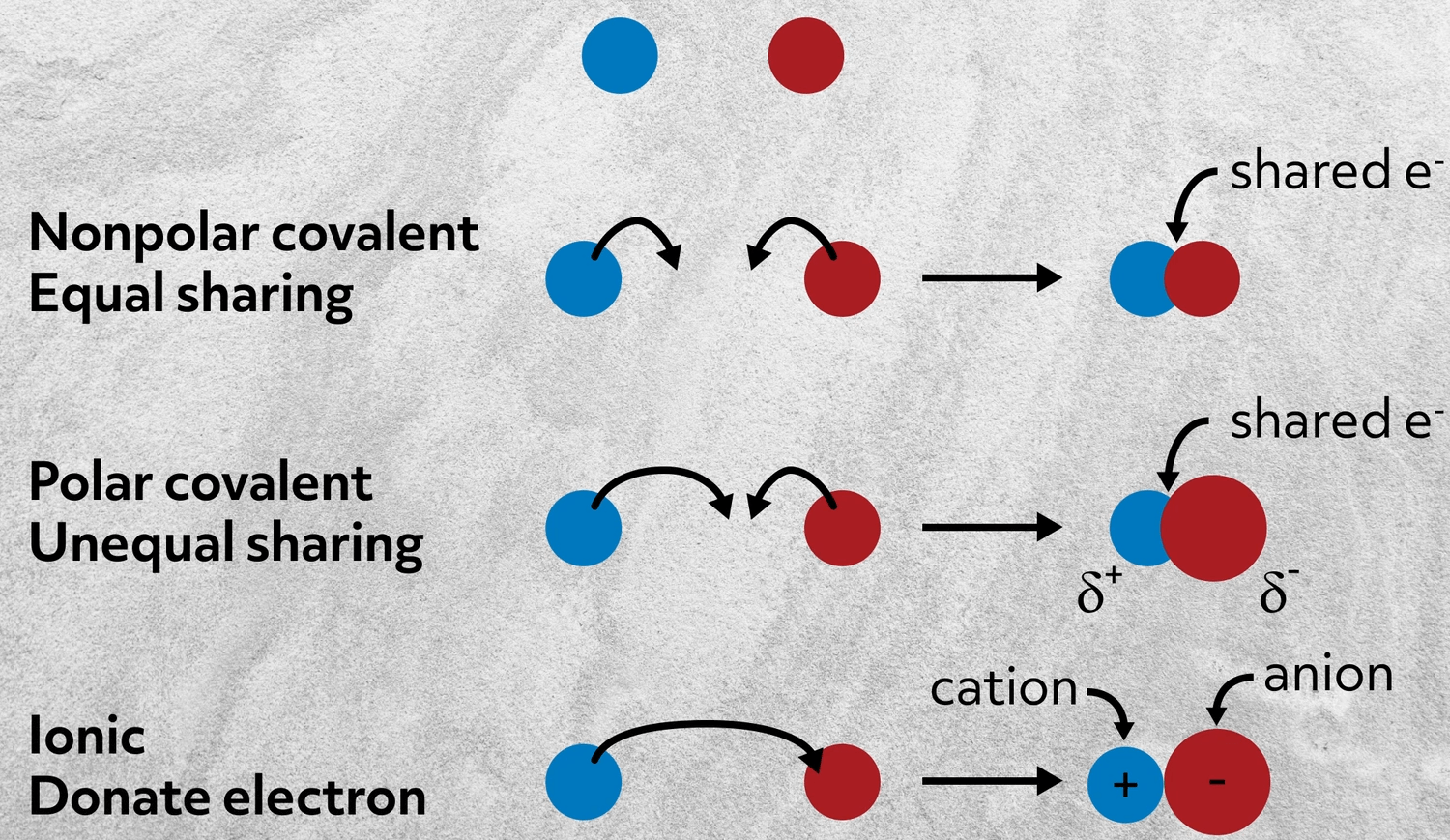
Covalent bonds form between two nonmetals.
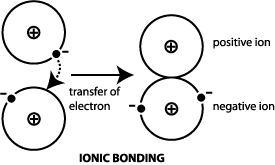
. It will produce a new substance. During chemical changes an ionic bonding forms when atom give away its electrons from another atom. Which of the following statements best describes metallic bonding.
No other bonding involves the formation of. The sharing of valence electrons between two or more neutral atoms. Which statement best describes an ionic bond.
The repulsion of ions due to the transfer of valence electrons B. See answer 1 Best Answer. A aspartate b glycine c serine.
Metals usually give up electrons and nonmetals usually accept electrons. Molecules repel one another due to intermolecular forces. No answer text provided.
Two atoms sharing a set of electrons when two elements with same charge are held together by electrostatic forces Baton atoms exchanging a set of electrons COne atom giving up some of its electrons to another atom ENone of the above. The sharing of a pair of electrons between two atoms a relatively strong bondb. Which characteristics best describes the physical change of water.
The only pure covalent bonds occur between identical atoms. A two atoms sharing a set of electrons B two atoms exchanging a set of electrons C one atom giving up some of its electrons to another atom D when two elements with same charge are held together by electrostatic forces. Make an illustration on the following objects to show how sound energy is.
An ionic bond involves a metal that transfers one or more electrons to a nonmetal. The attraction of ions due to the transfer of valence electrons D. Otwo atoms sharing a set of electrons when two elements with same charge are held together by electrostatic forces Otwo atoms exchanging a set of electrons O one atom giving up some of its electrons to another atom none of the above.
- 11353902 uriecandelaria. What best describes the bonding in a water molecule. The attraction between two charged atoms a relatively weak bond in an aqueoussolution.
Electrons are transferred from one atom to another. An ionic bond involves two nonmetals that share electrons. Which of the following best describes ionic bonding.
Water is essential in the body. The sharing of a hydrogen atom between two other atoms a relatively weak bond. Ionic bonds always take place between a metal and nonmetal.
Which results in positive and negative ions. Just think of positive and negative charges attracting to one another thats really all an ionic bond is. Ionic bonds form so that the outermost energy level of atoms are filled.
The sharing of a pair of electrons between two atoms a relatively weak bond. Which of the following best describes ionic bonding. See answer 1 Best Answer.
A A compound that contains the element lead. There is always an octet of electrons around an atom in a molecule. Strong forces of attraction between positively charged metal ions and.
Which of the following best describes ionic bonding. What statement best describes ionic bonding. Ionic bonds is a bond that forms when electrons is being transferred from one form to another form.
It is called an ionic bond because when an element gives up or accepts electrons it gains either a positive or negative charge respectively. Which of the following best describes ionic bonding. Identify which of the following amino acids has a side chain that may be important in binding a drug by ionic bonding.
The transfer of valence electrons between atoms to make them neutral C. An ionic bond essentially donates an electron to the other atom participating in the bond while electrons in a covalent bond are shared equally between the atoms. Aone atom giving up some of its electrons to another atom bwhen two elements with same charge are held together by electrostatic forces ctwo atoms exchanging a set of electrons dtwo atoms.
Which of the following statements best describes a lead compound. An oxygen atom shares an electron pair with each H atom. The ionic bond as its name suggests is most easily characterized by the formation of charged ions.
It behaves like most other molecules in all ways. Non-metals share electrons to become stable forming a compound whose representative particle is a molecule C. Electrons are attracted to the nucleus of the central atom.
Which of the following describe ionic bonding. One atom giving up some of its electrons to another atom two atoms sharing a set of electrons two atoms exchanging a set of electrons when two elements with same charge are held together by electrostatic forces. Which of the following is NOT an ionic compound.
Question 29 Which of the following best describes ionic bonding. An oxygen atom shares a single electron with each H atom. Shared and unshared electron pairs repel each other as much as possible.
Ionic bonds form between a metal and a nonmetal.

Ionic Compounds Bonds Structure Properties 1 6 5 Edexcel Igcse Chemistry Revision Notes 2019 Save My Exams

The Ionic Bond Boundless Chemistry
0 Comments